As r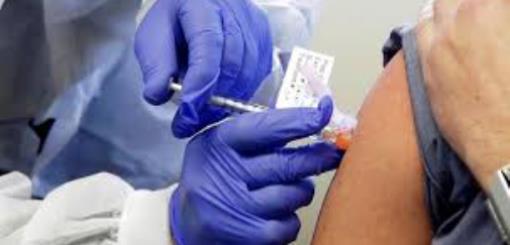 eported by CNN, only six countries in Latin America are currently conducting clinical trials or plan to participate in any.
eported by CNN, only six countries in Latin America are currently conducting clinical trials or plan to participate in any.
Argentina is one of them and has already authorized eight clinical trials of vaccines against coronavirus, in addition to 22 clinical studies on drugs.
The report quotes infectologist Omar Sued, chairman of the Argentine Society of Infectious Diseases, who considers that the country offers "several advantages: experience in the field of research, a serious regulatory agency such as ANMAT, that is considered a reference by the WHO".
Currently, six phase-three studies are being carried out in the country. Phase three is when researchers seek to confirm the vaccine's efficacy and safety on a large scale.
The first jab to be approved, let's recall, was that of Pfizer and BioNtech, back in July 2020, which was the most comprehensive, as it included the first three phases of research. Then came the trials of Sinopharm and Johnson & Johnson's vaccines.
So far in 2021, two trials have already been authorized: first, that of Merck, Sharp & Dohme (MSD), which has just been cancelled by the laboratory for unsatisfying preliminary results, and then one for CureVac by Bayer.
In October 2020, Argentine authorities gave the go-ahead to trials of a vaccine developed in Spain: Ruti.






